Our Technology
Engineering Fit-for-Purpose Biotherapeutics
Our technology leverages Zymeworks’ industry leading expertise in the fields of protein engineering and drug chemistry to discover and develop the next generation of antibody-based therapeutics to combat unmet medical need in hard-to-treat cancers and other serious diseases.
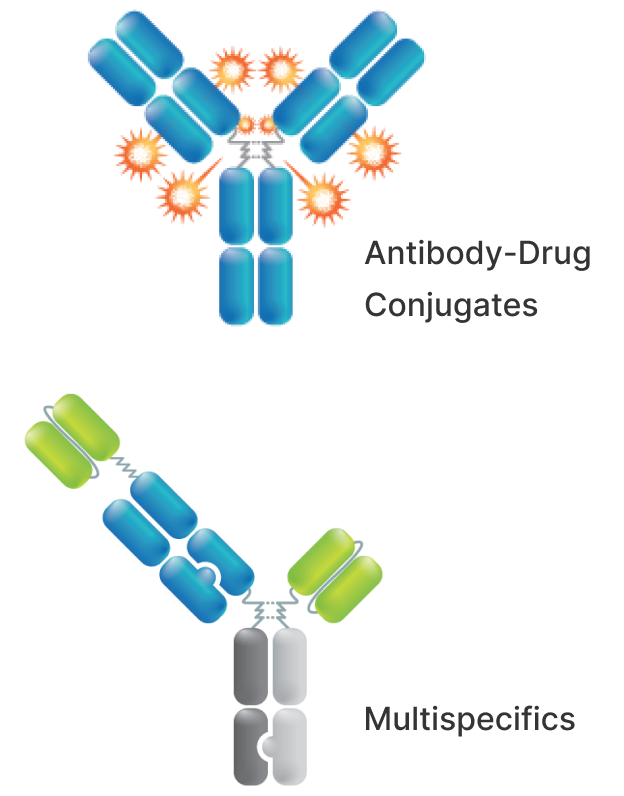
The integration of complementary and antibody-based technologies, embedded in our proprietary Multispecific and Antibody-Drug Conjugate (ADC) therapeutic modalities, interfaced with disease biology, enables the development of differentiated and fit-for-purpose therapeutics.
Select Difficult-to-Treat Cancers & Target

Design with Complementary Technology
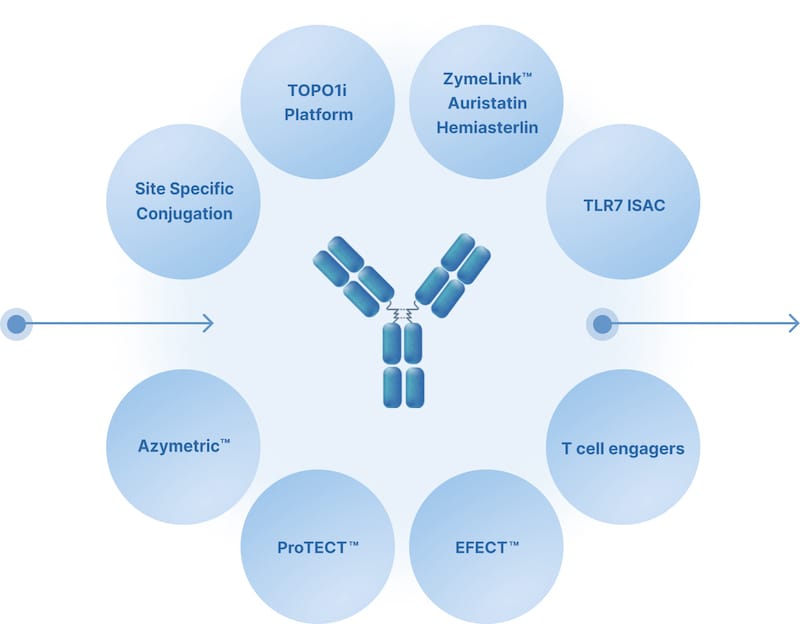
Optionality with Two Foundational Fit-for-Purpose Modalities
Zymeworks is an industry leader in both multispecific research and the use of this technology in developing therapeutics targeting areas of high unmet medical need.
Our clinically validated technologies harness the flexibility of our proprietary AZYMETRIC™ platform as a foundation to solve biological challenges.
Explore our technologies
We are dedicated to the development of highly differentiated ADC therapeutics that will make a real difference for people impacted by difficult to treat cancers and other diseases.
ADCs are antibodies modified to deliver a toxic drug, known as a payload, directly into the cancer cell through binding to a specific target expressed on tumor cells recognized by the antibody. Payloads are selected and engineered to match disease biology with payload mechanism. Successful development of ADCs requires the right combination of target, antibody, linker, conjugation, and payload.
Our unique approach to the design and development of ADCs paired with our toolbox of ADC and protein engineering technologies provides us with the flexibility to precisely engineer and develop highly differentiated ADC therapeutics.
Target: Favorable expression profile in cancers with greatest unmet need
Our Thinking:
We are focused on cell surface targets in populations with the greatest unmet patient need. Our team selects novel targets and targets with evidence of clinical activity to develop fit-for-purpose ADCs.
Our Targets include:
- human epidermal growth factor receptor 2 (HER2) (zanidatamab zovodotin)
- Folate Receptor Alpha (FRa) (ZW191)
- Glypican-3 (GPC3) (ZW251)
- NaPi2b (ZW220)
Antibody: Development of optimal ADC antibodies
Our Thinking:
Antibodies specifically selected for ADC use can increase the likelihood of developing a successful ADC. We leverage internal antibody discovery expertise to select antibodies with superior internalization, payload delivery, and tumor penetration for use in our ADC product candidates. Additionally, we use our Azymetric™ technology to create biparatopic and bispecific antibodies that may improve internalization, specificity, and lower the target expression threshold for our ADCs.
Linker & Conjugation: Tailored to desired ADC mechanisms and payload characteristics
Our Thinking:
Conjugation chemistry and linker technology can improve preclinical maximum tolerated dose (MTD) through increased stability, but this approach is not necessarily supported by clinical evidence. Site specific conjugation can enable optimization of ADC biophysical characteristics and precision tailoring of drug to antibody ratio where required. We believe that conjugation chemistry and linker design should be tailored to payload mechanism, site of action, biophysical characteristics, pharmacokinetics, and potency. Antibody target biology and structural characteristics are considered as part of a holistic design approach for linkers and ADCs.
Our Linker/Conjugation:
- Our proprietary cysteine-insertion conjugation platform is leveraged where appropriate
- Stochastic cysteine and lysine-based conjugation methods are generally suitable
Payload: Match disease and target biology with payload mechanism
Our Thinking:
Payloads should be matched with antibody targets based on tumor type, target biology, and antigen expression. Payload potency dictates platform tolerability and determines if ADCs can be dosed at sufficient levels to overcome exposure limitations of on-target off-tumor disposition. Exploring payloads with better drug-like properties (such as solubility, permeability, metabolic stability, and transporter substrate profile) may improve clinical ADC attrition rates.
Our Payloads:
- Auristatin (ZD02044)
- Topoisomerase 1 inhibitor (ZD06519)
- Hemiasterlin (ZD01886)
- TLR7 agonist ISAC (immunostimulatory drug conjugates)